*Disseminated on Behalf of Medicus Pharma Ltd.
Market Crux Initiates Coverage On (NASDAQ: MDCX) Starting This Morning—Tuesday, September 23, 2025 (MDCX) Comes Backed By Several Potential Catalysts—And Here's What
We Can Tell You So Far…
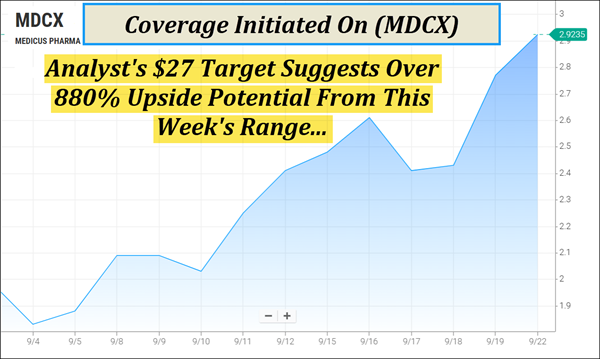
Recent Momentum: (MDCX) Has Moved Approximately 67% In Three Weeks And Now Trends Above Its 5, 20, And 50-Day Moving Averages.
Analyst Coverage: Targets As High As $27 Have Been Reported For (MDCX), Which Suggest Over 880% Upside Potential From This Week's Range.
Strong Insider Ownership: More Than 75% Of (MDCX) Shares Are Held By Insiders, Aligning Leadership Closely With Company Direction.
Small Float: With A Small Float Of Less Than 3M Shares, (MDCX) Could
Witness The Potential For Big Moves If Demand Starts To Shift. Pull Up (MDCX) While It's Still Early…
September 23, 2025
Trend Watch | (NASDAQ: MDCX) Eyes Key Technical Level On Early Move Dear Reader, We're watching the trend on (MDCX) right now. (MDCX) tapped $2.99 in the early session, eyeing a key technical level with its 100-Day moving average hovering at $3.17 according to Barchart's analysis tool. Medicus Pharma Ltd. (NASDAQ: MDCX) is pressing forward in areas where better solutions are urgently needed, and those early signals are starting to stack. With an approximate 67% move in just three weeks, (MDCX) is beginning to draw a sharper focus on what could come next. The chart now shows the company trending above several key technical levels — including its 5, 20, and 50-day moving averages. Together, these signals suggest the potential for continued momentum. And following its recent acquisition, (MDCX) is topping our watchlist this morning—Tuesday, September 23, 2025. But keep in mind, (MDCX) has an ultra-small float, with fewer than 3 Mln shares listed as available to the public according to FinViz. Insider ownership is also reported at over 75%, a level that could reflect a management team's confidence in the company's direction. Recent Analyst Target Suggests Over 880% Upside Potential
Multiple news sites and market portals, including Benzinga, MarketWatch, and TipRanks, have reported that D. Boral Capital analyst Jason Kolbert recently set a $27 target on (MDCX), which suggests over 880% upside potential from its recent $2.75 range. That's not the only target we're tracking. In coverage released Thursday, Maxim Group analyst Jason McCarthy reiterated a $20 target for (MDCX) — pointing to significant upside from where shares recently traded. His outlook is supported by a pipeline that continues to advance in areas where new treatment options are urgently needed. At the forefront is a program in development that could redefine how prostate cancer is addressed. Antev Acquisition Adds Two Therapeutic Programs To (MDCX)

Medicus Pharma (NASDAQ: MDCX) has completed its acquisition of Antev Limited, gaining control of Teverelix trifluoroacetate, a next-generation GnRH antagonist in late-stage development for both acute urinary retention (AURr) and high cardiovascular-risk prostate cancer. The move broadens (MDCX)'s clinical scope into two therapeutic areas with a combined $6B market potential.
Teverelix is designed for dual use: in AURr, a condition with high recurrence rates, it aims to become the first product to prevent relapse, supported by an FDA-cleared Phase 2b study of 390 patients. In prostate cancer patients with elevated cardiovascular risk, it is being evaluated in another FDA-cleared Phase 2b trial as a potentially safer alternative to conventional therapies. The acquisition also adds leadership depth, with veteran pharma executive Patrick J. Mahaffy joining the board. Together, the expanded pipeline and strengthened leadership put (MDCX) in position to reshape care across two areas of pressing medical need. (MDCX) Expands Clinical Footprint With SkinJect Trials In U.S. And UAE
Beyond its urology pipeline, (MDCX) is advancing SkinJect, a dissolvable microneedle patch for non-melanoma skin cancers like Basal Cell Carcinoma (BCC). It bypasses surgery, delivering doxorubicin directly into the lesion to target tumor cells and trigger an immune response that may reduce recurrence. BCC remains one of the most common cancers diagnosed annually in the United States, and with traditional treatments often invasive, the demand for less disruptive options continues to rise. On September 8, 2025, (MDCX) announced the launch of its SKNJCT-004 Phase 2 study in the United Arab Emirates, with patient recruitment now underway at Cleveland Clinic Abu Dhabi. Additional enrollment is planned across Sheikh Shakbout Medical City, Burjeel Medical City, Rashid Hospital, Clemenceau Medical Center, and American Hospital of Dubai. The trial will evaluate two dose levels of the patch in a randomized, double-blind, placebo-controlled design enrolling 36 participants. This new study builds on (MDCX)'s SKNJCT-003 Phase 2 trial in the United States, which has already randomized more than 75% of its expanded 90-patient enrollment following a positively trending interim analysis earlier this year. Together, the U.S. and UAE programs highlight (MDCX)'s commitment to advancing SkinJect as a potential first-in-class, non-invasive therapy for skin cancer across global healthcare systems. Why Medicus Pharma (NASDAQ: MDCX) Stands Out
With two late-stage clinical programs now in motion, expanding partnerships, and a lean structure compared to its potential, (MDCX) has earned a spot on our radar. The company is approaching a critical stretch, with progress in the clinic and key regulatory steps expected ahead. For those tracking biotech catalysts, (MDCX) is increasingly difficult to ignore. Momentum is stacking across its pipeline and leadership, setting it apart from sector peers. When we lined up the numbers, the strategy, and the science, seven clear factors emerged that show why this morning could be a turning point. Here's 7 Reasons Why (MDCX) Is Topping Our Watchlist
This Morning—Tuesday, September 23, 2025
1. Analyst Coverage: Recent coverage on (MDCX) has targets as high as $27, which suggests over 880% upside potential from its current range. 2. Small Float: Fewer than 3M shares of (MDCX) are listed as available to the public, creating conditions where shifts in demand can have amplified effects. 3. Recent Momentum: Over the past three weeks, (MDCX) has moved approximately 67% and is now trending above its 5, 20, and 50-day moving averages.
4. Strong Insider Ownership: With insider ownership above 75%, leadership at (MDCX) is deeply tied to the company's direction and progress. 5. Expanded Pipeline: Following the Antev acquisition, (MDCX) now controls Teverelix, a Phase 2 asset in both acute urinary retention and high cardiovascular-risk prostate cancer. 6. International Clinical Strategy: Phase 2 clinical studies for SkinJect are running in parallel across the U.S. and UAE, positioning (MDCX) on an international development path. 7. Boardroom Depth Expands: The addition of Patrick J. Mahaffy, a seasoned executive with prior CEO roles in oncology, strengthens the strategic leadership of (MDCX).
All seven factors point to one conclusion—(MDCX) is entering a decisive stretch. With multiple potential catalysts aligning across its pipeline, we have all eyes on (MDCX) this morning. Pull Up (MDCX) While It's Still Early…
With momentum stacking — an approximate 67% move in recent weeks and strength now showing above key technical levels — (MDCX) has forced its way onto our radar. Analyst targets remain aggressive with one suggesting over 880% upside potential, and insider ownership above 75% underscores leadership alignment with the company's direction. Layer in the razor-thin float, the completed Antev deal that brought Teverelix into late-stage development, and SkinJect trials progressing across both the U.S. and UAE, and the setup becomes even harder to ignore. Now, with experienced leadership steering the next phase, (MDCX) is entering a stretch that demands close attention. All eyes on (MDCX) this morning. Make sure you pull (MDCX) while it's still early. Keep an eye out for my next update, it could be here any moment. Sincerely, Gary Silver
Managing Editor, Market Crux
| 



