*Sponsored
Gary Silver Reinitiates Coverage On Actuate Therapeutics, Inc. (NASDAQ: ACTU) Starting This Morning—Friday, June 20, 2025
And Here's Why…
Analyst Targets Raised: New $20 And $32 Targets Suggest 160%+ And 320%+ Potential Upside.
Low Float: Fewer Than 6M Shares Could Set The Stage For Potential Big Swings If Demand Begins To Shift.
Insider Strength: 65%+ Insider Ownership Signals Strong Internal Conviction.
Momentum Shown: (ACTU) Moved Approx. 50% Since The First Time
We Brought It To You.
Phase 2 Data: Survival Benefit Reported At A Top Global Oncology Meeting.
Index Addition: Set For Russell 3000® And 2000® Inclusion, Boosting Visibility.
Regulatory Progress: Pre-NDA Discussions With FDA Planned,
Advancing To Next Milestones.
Pull Up (ACTU) While It's Still Early
June 20, 2025 Friday's Early Watchlist: Coverage Reinitiated On (ACTU) Starting Right Now Dear Reader, Some companies grab headlines. Others quietly work on breakthroughs that could change the game. This morning, one company is stepping out from under the radar and into the spotlight — tackling one of modern medicine's toughest challenges and delivering real-world results where many have struggled. That company is Actuate Therapeutics, Inc. (NASDAQ: ACTU) — and if you haven't been watching closely, this is the moment to change that. 
According to several news sites and portals, including Benzinga, Barchart, and TipRanks, H.C. Wainwright's Dr. Swayampakula Ramakanth recently reiterated a $20 target on this name, suggesting over 160% potential upside from Wednesday's $7.49 close. Craig-Hallum's Albert Lowe went even further — raising his target to $32, pointing to over 320% potential upside, and reaffirming his bullish stance on the company. This is backed by more than projections. The company has fewer than 6M shares in the float, paired with over 65% insider ownership — a combination that could lead to the potential for big moves if demand begins to shift. It's not just about structure. The catalyst behind this attention is positive Phase 2 data shared at one of the world's top oncology conferences, reporting meaningful survival benefits in metastatic pancreatic cancer — a space where progress has been elusive. And since we last highlighted this profile on February 25, it has already made an approximate 50% move, climbing from $7.99 to $11.99 by May 30. That move didn't happen by chance — it reflects growing momentum around meaningful milestones. Why Actuate Therapeutics Is Stepping Into the Spotlight Now…

With a rare combination of momentum, structure, and analyst confidence, (ACTU) is stepping into the spotlight. The attention isn't just about numbers — it's about what's driving them and the mission behind the company's work. Let's take a closer look at what (ACTU) is building, and why it's capturing so much interest right now: Actuate Therapeutics, Inc. (NASDAQ: ACTU) is a clinical-stage biopharmaceutical company dedicated to developing therapies for some of the most difficult-to-treat and high-impact cancers. The company's mission is to create meaningful new options for patients facing aggressive tumors where current treatments offer limited benefit — with a particular focus on metastatic pancreatic cancer, metastatic melanoma, refractory Ewing sarcoma, and other resistant cancers. At the center of (ACTU)'s work is elraglusib, a potentially class-leading GSK-3β inhibitor with a novel, multimodal mechanism designed to disrupt multiple cancer survival pathways, enhance immune response, and increase tumor sensitivity to both chemotherapy and immunotherapy. 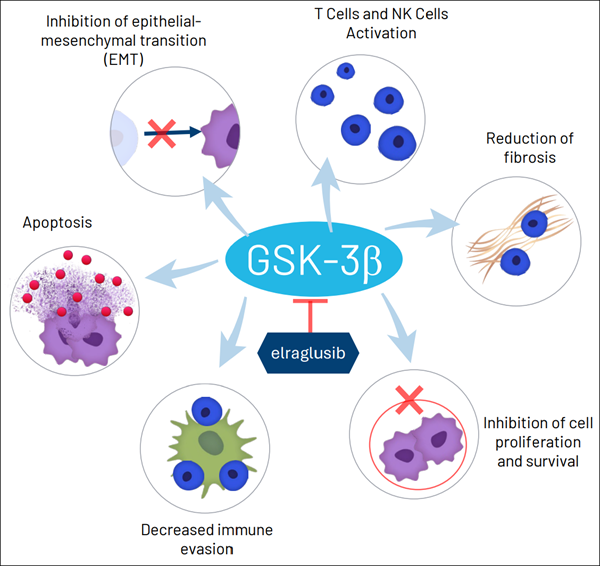
This approach aims to overcome resistance and improve outcomes where standard therapies have fallen short. (ACTU) is advancing elraglusib in both intravenous and oral formulations across multiple clinical programs. This includes a Phase 2 trial in metastatic pancreatic cancer, where elraglusib combined with gemcitabine/nab-paclitaxel (GnP) delivered a clinically meaningful survival benefit — extending median survival from 7.2 months to 10.1 months in previously untreated patients. 
These positive results were recently presented at the American Society of Clinical Oncology's (ASCO) annual meeting, one of the world's most respected cancer research gatherings, and have drawn significant attention in the field. Beyond pancreatic cancer, (ACTU) is exploring elraglusib in additional solid and hematologic tumors, with a clear focus on diseases with urgent unmet needs. The company's efforts are supported by a robust global patent portfolio, multiple regulatory designations (including Orphan Status and Fast Track status for pancreatic cancer), and a leadership team with decades of experience in developing and commercializing oncology therapies. With pre-NDA discussions planned before year-end, analyst targets raised as high as $32, a tightly held share structure, and momentum continuing to build, it's clear why Actuate Therapeutics (ACTU) is topping our watchlist right now. Recent Developments…
Inclusion in Russell 3000® and Russell 2000® Indexes (June 16, 2025)
Actuate announced its upcoming inclusion in the Russell 3000® and Russell 2000® Indexes, effective June 27, 2025. This milestone reflects the company's continued momentum following its positive Phase 2 data in metastatic pancreatic cancer. CEO Daniel Schmitt called this a pivotal moment that elevates Actuate's visibility and reinforces confidence in its strategic direction. Key Opinion Leader Event on Positive Phase 2 Data (June 2, 2025)
Actuate shared highlights from its KOL event after presenting positive Phase 2 data at ASCO. Top experts from leading cancer centers emphasized elraglusib's survival benefit, unique immune-modulating mechanism, and potential to reshape mPDAC treatment. The company reported a favorable safety profile and confirmed plans for advancing regulatory discussions with the FDA and EMA. What's Next for This Mission…
This isn't about chasing milestones or checking boxes. This is about reshaping what's possible — and that's why (ACTU)'s next steps are being watched so closely. (ACTU) is preparing for a pre-NDA meeting with the FDA later this year, setting the stage for regulatory discussions that could move elraglusib closer to broad clinical use. And Actuate isn't stopping at pancreatic cancer. With ongoing research into refractory Ewing sarcoma, metastatic melanoma, and an oral version of elraglusib in the works, this is a company building for the future of cancer care on multiple fronts. Every move is part of a larger mission: to give patients where time is precious a new reason to fight. And that's exactly why (ACTU) may not be flying under the radar for much longer — it's moving onto the screens of those watching for real-world momentum and breakthrough potential. With powerful data, key milestones ahead, and attention building fast, this could be a critical time to take a closer look. Here's why (ACTU) just hit the top of our watchlist this morning — and why you might want to keep it on yours: 7 Reasons Why (ACTU) Is Topping Our Watchlist This Morning—
Friday, June 20, 2025…
1. Analyst Targets Raised: analysts tracking (ACTU) have reiterated or increased their price targets following the latest data and developments, including a $20 target (over 160% potential upside) from H.C. Wainwright and a $32 target (over 320% potential upside) from Craig-Hallum.
2. Ultra Low Float: fewer than 6M shares in the float means (ACTU) has the potential to witness significant swings if demand begins to shift due to its limited supply. 3. Insider Confidence: over 65% insider ownership at (ACTU) reflects a high level of internal belief in its direction and mission. 4. Recent Momentum: since the last time we brought you (ACTU) on February 25, it has made an approximate 50% move, showing how momentum can build around milestones. 5. Positive Phase 2 Data: (ACTU) reported a clinically meaningful survival improvement in metastatic pancreatic cancer at one of the world's top oncology conferences. 6. Index Inclusion: (ACTU) is being added to the Russell 3000® and Russell 2000® Indexes, reflecting broader recognition and visibility in the market. 7. Upcoming Regulatory Steps: (ACTU) is preparing for pre-NDA discussions with the FDA, marking active progress toward key next-stage milestones. All of this points to a company that's no longer quietly working in the background — (ACTU) is stepping into the spotlight. With attention building fast, now could be the moment to pull this up before the next headline hits. Pull Up (ACTU) While It's Still Early…
Actuate Therapeutics, Inc. (NASDAQ: ACTU) just landed on our radar, and there's plenty to take note of this morning. (ACTU) could start drawing attention with its ultra-low float of under 6M shares, over 65% insider ownership, and the fact that it made an approximate 50% move since the last time we brought it to you. Analysts have raised targets as high as $32 following positive Phase 2 data in metastatic pancreatic cancer, and the company's inclusion in the Russell indexes signals growing recognition. We have all eyes on (ACTU) this morning. Take a look at (ACTU) while it's still early. Keep an eye out for my next update—it could be here within the hour.
Sincerely, Gary Silver
Managing Editor, MarketCrux | 





